Biotechnologický ústav AV ČR
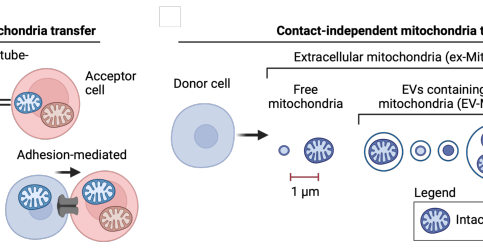
Horizontální mitochondriální přenos: Nový podobor buněčné biologie
Horizontální mitochondriální přenos (HMT) je proces, při kterém se mitochondrie včetně své DNA přesouvají mezi buňkami, což může významně ovlivnit jak přijímající, tak i dárcovskou buňku. Tento jev je poměrně nedávno rozpoznaný, první zmínky o něm se objevují v 90. letech minulého století.

Instruct-ERIC jednal v BIOCEV o budoucnosti infrastruktur strukturní biologie
Vestec, Česká republika – Ve dnech 21. a 22. listopadu se v centru BIOCEV sešli zástupci výzkumné infrastruktury Instruct-ERIC, aby diskutovali o současnosti a budoucnosti strukturní biologie. Setkání, které hostil Biotechnologický ústav (BTÚ), přivedlo dohromady přední evropské výzkumníky poskytující přístup k nejmodernějším technologiím a metodám v oboru, aby společně prozkoumali budoucí směřování a priority evropských infrastruktur.
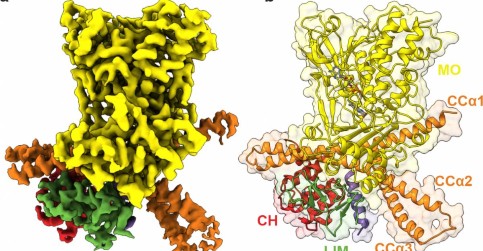
Na dynamiku buněk má zásadní vliv protein MICAL1, kontroluje buněčný cytoskelet
Vědci dosáhli významného pokroku v pochopení toho, jak buňky regulují svou vnitřní strukturu. Tým ze tří ústavů Akademie věd České republiky, Ústavu molekulární genetiky, Biotechnologického ústavu a Ústavu organické chemie a biochemie, z Přírodovědecké fakulty Univerzity Karlovy, BIOCEV a CEITEC odhalil molekulární mechanismy proteinu MICAL1, který je klíčový pro udržování tvaru a pohybu buněk. ...

MOSBRI konference řešila výzvy v oblasti správy dat v biofyzice
Vestec, Česká republika — Minulý týden hostil Biotechnologický ústav konferenci MOSBRI v centru BIOCEV, kde se sešli přední vědci, odborníci na datové úložiště a biofyzikové, aby řešili klíčové výzvy v oboru: efektivní správu a dostupnost dat. Dvoudenní akce, která se konala 7. a 8. listopadu, podpořila diskuse o pokrocích v interoperabilitě a integrativních přístupech ke sdílení a analýze dat.
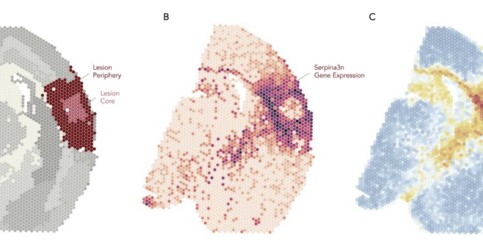
Průlomová studie mapuje proces zotavení mozku po mrtvici
Tým vědců z Biotechnologického ústavu Akademie věd ČR (BTÚ AV ČR) a Ústavu experimentální medicíny Akademie věd ČR (ÚEM AV ČR) s podporou kolegů z Institutu klinické a experimentální medicíny (IKEM) učinil významný pokrok v pochopení toho, jak se mozek zotavuje po mrtvici. ...






